MEDICAL APPLICATIONS: Lensless microscopy system reveals 3-D structures
To reduce the cost of point-of-care analysis for patients in remote locations,microscopy systems must be both portable and easily accessible. To lower the cost of such instruments while providing 3-D images of water-borne parasites, blood-borne parasites, and diseases involving blood cell deformations, PhD student Guoan Zheng of the California Institute of Technology (Pasadena, CA, USA; www.caltech.edu) has developed an on-chip microscope that provides high-resolution images while eliminating the need for bulky and expensive lenses.
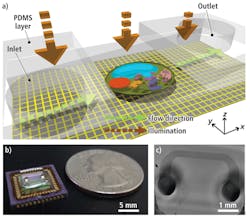
Known as a sub-pixel resolving optofluidic microscope (SROFM), the system uses a microfluidic channel placed directly above the surface of a MT9T001 CMOS image sensor with a 3.2-μm pixel size from Aptina (San Jose, CA, USA;www.aptina.com). This 1.5 × 1.5-cm channel is 50–300 µm wide with a height of 15–27 µm and is attached to the imager at an angle between 10° and 30°.
Before the color imager could be used in the device, however, it was necessary to remove the Bayer color pattern and the microlens layer from the device by exposing the sensor under oxygen plasma for 10 min. Once this layer was removed, the sensor was soldered onto a Silicon Video 9T001C camera system fromEpix (Buffalo Grove, IL, USA; www.epixinc.com) and images from the camera transferred to a host PC using a PIXCI SI digital frame grabber also from Epix.
In operation, a liquid containing target samples such as cells is introduced into the inlet channel of the microfluidic channel and capillary action induces a flow in the channel, moving the samples across the sensor pixel grid of the CMOS sensor (see Fig. 1). These samples were imaged at a flow velocity of 200 µm/s, with a camera frame rate of 400 frames/s and a field of view (FOV) across the microfluidic cell of 250 µm × 2 mm.
Although the maximum resolution of the system would normally be that of twice the Nyquist sampling rate or approximately 6.4 µm, Zheng and his colleagues used a multiframe pixel approach to increase this resolution. To accomplish this, a series of low-resolution images is first captured and the motion vector of the low-resolution series of images computed by analyzing the position of the sample as it moves across the low-resolution image sequence.
Once this motion vector is computed, a shift-and-add algorithm is applied to reconstruct a single high-resolution image. This algorithm consists of shifting each low-resolution image by the relative sub-pixel shift provided by the computed motion vector and adding all the images to form a high-resolution version. Wiener deconvolution is then used to remove pixel blurring in the final high-resolution image.
To establish the extent of the resolution improvement using this technique, Zheng and his colleagues imaged a solution containing 0.5-µm microspheres. These tests showed that the system has a resolution limit of approximately 0.75 µm and that adjacent particles can be resolved as long as they are three or more high-resolution pixels apart. Using a conventionalmicroscope with a 20X objective lens, the achievable resolution would be 0.84 µm (see Fig. 2).
FIGURE 2. By using a series of low-resolution images and computing a motion vector between them as they move across the microfluidic plate, a series of high-resolution images can be created. These are comparable in resolution to those obtained with a microscope.
As well as providing comparable resolution to lens-based systems, the SROFM can image samples with rotational movement, providing researchers with the opportunity to image samples from different perspectives. When cells rotate while flowing through the channel, the cells can be imaged and their 3-D structures determined. Better still, high-resolution video images of these samples can also be captured as they flow through the channel and interact with the fluid.
Vision Systems Articles Archives