Optical sensor tracks zinc in cells for cancer research
Chemists from MIT have developed an optical sensor that can be added and diffused into cells and used to track zinc within the cells, which may help researchers learn more about its function and role in prostate cancer.
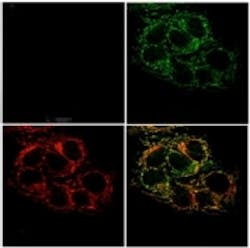
In a research paper published in the Proceedings of the National Academy of Sciences, the team describes their work on the sensor, which relies on a molecule first developed in Stephen Lippard, senior author of the research paper and MIT chemistry professor, and is known as Zinpyr1 (ZP1). It is based on a dye known as fluorescein, but it is modified to fluoresce only when it binds to zinc, according to MIT.
ZP1 is targeted to specific organelle within a cell, and when bound with zinc, fluoresces to enable the scientists to determine where the metal is concentrated. This is particularly useful for the study of prostate cancer, due to the fact that zinc levels drop dramatically in cancerous prostate cells. As a result, the team is able to study zinc trafficking within the prostate cells, both healthy and diseased, and by doing so; they are able to gain insight into how zinc levels within the cell change during the progression of prostate cancer, according to Robert Radford, MIT postdoc, project leader, and co-author of the research paper.
Zinc helps stabilize protein structure and catalyzes some cellular reactions. Scientists have theorized that when the prostate cells become cancerous, they banish zinc from mitochondria, which allows the cancer cell to produce the extra energy in needs to grow and divide. This new study supports this theory by showing that, although cancerous prostate cells can absorb zinc, the metal does not accumulate in the mitochondria, as it does in normal prostate cells. As a result, zinc in normal cells is likely transported into mitochondria by a specialized transport protein, but such a protein has not been identified, according to the press release. In cancer cells, however, this protein might be inactivated.
In future studies, the team plans to expand their strategy to create sensors that target many other organelles in the cell.
"The identification of intracellular targets for mobile zinc is an important step in understanding its true function in biological signaling. The next steps will involve discovery of the specific biochemical pathways that are affected by zinc binding to receptors in the organelles, such as proteins, and elucidating the structural and attendant functional changes that occur in the process," Lippard said in the press release.
View the MIT press release
Also check out:
MIT develops hand-held scanner for detecting eye disease
MIT and Massachusetts General Hospital developing new X-ray imaging technology
3D imaging and analysis software detects lung cancer in sputum cells
Share your vision-related news by contacting James Carroll, Senior Web Editor, Vision Systems Design
To receive news like this in your inbox, click here.
Join our LinkedIn group | Like us on Facebook | Follow us on Twitter | Check us out on Google +
About the Author

James Carroll
Former VSD Editor James Carroll joined the team 2013. Carroll covered machine vision and imaging from numerous angles, including application stories, industry news, market updates, and new products. In addition to writing and editing articles, Carroll managed the Innovators Awards program and webcasts.