Biomedical imaging discovers new MOLECULAR PARAMETERS
Biomedical imaging discovers new MOLECULAR PARAMETERS
By Lawrence J. Curran, Contributing Editor
Researchers working at a Canadian university are investigating molecular chemistry and cell structures with innovative imaging technology. Using a computer-controlled charge-coupled-device (CCD) camera to record their efforts in dynamic force spectroscopy (DFS), they can determine minute bone-ruptuure
minute bond-rupture forces, measured in picoNewtons (pN), as they probe molecular complexes.
Working with nanoscale-to-micro scale robotic control on the stage of an inverted microscope at the University of British Columbia (UBC; Vancouver, BC, Canada), the research ers are probing single bonds to surface-mounted proteins, lipids, and carbohydrates--the three organic compounds that form the structure of living cells. Their aim is to determine the force required to break single bonds between a molecule on the probe and one on the surface over a range of time intervals from 0.001 to 100 seconds.
Heading the team is Evan Evans, professor of physics and pathology at UBC and professor of biomedical engineering at Boston University (Boston, MA). He and his associates are focusing their studies on understanding the strength of receptor-ligand bonds, which are the noncovalent bonds that govern the fundamental activity of living cells.
The DFS approach enables re search ers to relate bond-rupture forces to molecular chemistry and structure for the first time, according to Evans. Spawned scientifically from a Canadian Institute for Advanced Research program in the Science of Soft Surfaces and Interfaces, the applications to biology are funded in part by separate grants from the Canadian Medical Research Council and the US National Institutes of Health, which is striving to better understand the role of cell adhesion in inflammation, injury, and tumor metastasis.
Innovative probe
An innovative tool the UBC researchers have devised is a computer-controlled probe, which is held by a micro pipette tapered to just 1 to 2 µm in diameter at its end. Evans describes the complete system as an instrument that combines a sensitive force probe, nanoscale-to-micro scale manipulation by motorized micrometers coupled to piezoelectric translation, and high-speed video image processing performed at 1 kHz.
Evans says his team wanted to establish a method that would reveal the profile of prominent energy barriers, which determine the strength of single receptor-ligand bonds. They began their work testing biotin-(strept) avidin bonds that served as prototypes for early studies using atomic force microscopy. Those studies provided force values only on a single time scale of 0.001 s.
In the course of this work, the researchers learned that although biotin-(strept) avidin bonds are among the strong est noncovalent linkages in biology, the strengths of these bonds are greatly affected by thermal activation, which makes them appear strong or weak depending on how fast or slow they are loaded or pulled apart.
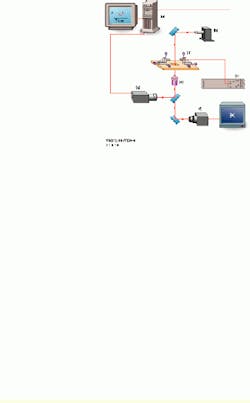
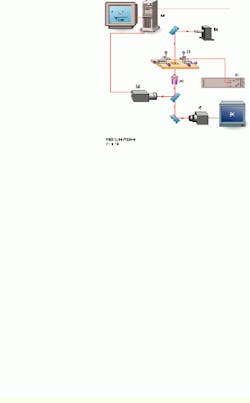
In the test process, the speed of loading is established by the spring constant of the tunable biomembrane force probe (BFP) and the velocity of probe-surface separation under computer control (see Fig. 1). The spring in the BFP is provided by a pressurized membrane capsule. Membrane tension establishes the force constant and is controlled by pipette suction. Using a red blood cell (dark shadow inside the end of the left pipette) as the transducer capsule, the BFP stiffness can be tuned between 0.1 and 5 pN/nm to measure forces from 0.5 to 2000 pN. A glass microbead of 1 to 2 µm in diameter is chemically glued to the membrane as the BFP tip. Maneuvered on the stage of an inverted microscope, the BFP is kept stationary, while the microbead test surface attached to the pipette on the right is moved to make soft contact with the BFP tip and is then pulled away from the tip using precision piezoelectric control.
The piezoelectric actuator can be moved at speeds measured in nano meters per second from 1 to 30,000. The simulated x-shaped cursor tracks the image of the probe tip throughout the cycle of approach-touch-separation by video image processing at 1000 frames/s, which yields a high-resolution discrimination of about 8 nm in position changes. Thus, with spring constants selected in a range from 0.1 pN/nm to 5 pN/nm, forces can be detected at the level of about 1 pN and measured up to 2000 pN.
Likewise, the speed of force application in the instrument can be controlled from the slowest rate of 0.1 pN/nm ¥ 1 nm/s = 0.1 pN/s to the fastest rate of 5 pN/nm ¥ 30,000 nm/s = 150,000 pN/s--an unprecedented six orders of magnitude. However, the useful limit to speed is set by the force of bond breakage at the fast rate and discrimination of rupture time, which is governed by the video framing speed.
For instance, with biotin-(strept) avidin bonds, the force reaches 200 pN at the fastest rate, which means that the bond lifetime is about 0.001 s and would not be noticed with standard 30-Hz video. Therefore, high-speed video processing is essential. At the slowest rate, biotin-(strept) avidin bonds live for about a minute and break at a very low force of about 5 pN, which is easily resolved by standard video.
Repetitive probing and retraction
To ensure that only single bonds are formed on touch, the surface concentration of molecules must be made extremely dilute so that only about one out of 10 touches results in a bond. "This requires that the process of approach-touch-separation be repeated for several hundreds of cycles, using computer-controlled piezoelectric displacement, to capture 50 to 100 rupture-force events at each decade in the six-order-of-magnitude range of loading rates," says Evans. In this way, the UBC researchers have been able to measure the rate dependence of bond-rupture forces over a far greater range than ever before possible, which is essential if force is to be related to molecular-scale interactions.
Andrew Leung, research scientist in the UBC department of pathology, points out that the combination of the bead and blood cell working as a "soft" force transducer enables the computer to sense (by force feedback) light touch to a surface and signal retraction or to push stronger and indent the surface structure if there is interest in the nanoscale compliance of the interface. "This delicate control of surface contact is important for the success of bond-force measurements and allows the rate of approach to surface contact to be set at a different speed than the rate of separation," adds Leung.
The high-speed video camera and the dynamic-link-library software were supplied by The Cooke Corp. (Tonawanda, NY). Using these tools, Kenneth Ritchie, at the time a UBC graduate research assistant in physics supervised by Evans, developed the software needed to extend the capability of the Cooke SensiCam CCD camera to operate in the 1000-frame/s range. In particular, the output of a custom PCI card was used to extract the image data needed to perform bead tracking. But, as Leung explains, instead of full frame, the image was masked to expose a central slice of the probe tip (glass microbead), which was collapsed to about three to five lines so that the camera acted similarly to a linescan device operating at 1 kHz.
Imaging design
Murad Karmali, Cooke product manager for SensiCam, says his company was initially contacted by UBC researchers with a request for help in tracking particles that move much faster than conventional imaging can capture at 30 frames/s. The researchers expressed an interest in the Cooke SensiCam CCD camera, which Karmali describes as a 12-bit fast-readout camera "that can provide a digital readout at video rates." But that speed wasn`t fast enough for the UBC researchers, and "they challenged us to give them the speed they needed even if we had to sacrifice a little in resolution," Karmali recalls.
Cooke engineers tried several approaches to boost speed, eventually settling on an innovative readout mode called fast framing (FFR). In FFR mode, hardware buffers collect data from a defined region of interest on the CCD, and the system ignores all but the desired data in that region, preventing the undesired data from corrupting the smaller set of desired data, Karmali says. "We effectively trick the analog-to-digital converter readout into reading only the selected data and to ignore the unwanted data."
Using FFR, the researchers can realize rates from 250 to 1000 frames/s. "The rate varies depending on the spatial resolution selected," says Karmali. For example, the conventional 30-frame/s rate yields a resolution of 640 ¥ 480 pixels. At 250 frames/s, the resolution decreases to 100 ¥ 100 pixels.
Cooke furnished the UBC re searchers with a model 360KL SensiCam, a PCI card, the FFR software, and a software developers kit. Cooke developed the SensiCam in conjunction with PCO Computer Optics GmbH (Kelheim, Germany). The two organizations were partners in developing the camera, which Cooke markets. The two firms also collaborated on developing the PCI card, which Cooke manufactures. The developers kit enables UBC to integrate camera-control software into the FFR routines.
The system computer is a board-level personal computer built around a P2B motherboard from ASUSTeK Computer International (Newark, CA), which provides a 300-MHz Intel Pentium II processor. The piezoelectric actuator moves under the control of a special high-voltage power supply, which, in turn, is controlled by a computer-based wave-generating board from Quatech (Akron, OH). The video output is processed through a Millennium II video board from Matrox Electronic Systems Inc. (Dorval, Quebec, Canada) and then by the dedicated Cooke PCI board for the high-speed camera.
Leung says the results of the imaging research have provided new insights into the chemical kinetics of single receptor-ligand bonds that are unobtainable otherwise. The UBC group can now measure six decades of loading rates, ranging from very slow to very fast. In addition, the speed of force application has been raised from 0.1 pN/s in six tenfold steps to reach approximately 100,000 pN/s, where detection of bond lifetime is unattainable with a conventional video camera. And, the fast range above 1000 pN/s was previously unobservable and would have remained so without the use of the SensiCam and the software developed by Ritchie, says Evans.
Dynamic force spectroscopy, Evans concludes, "opens up a new realm of investigation into weak biochemical interactions where, for the first time, measurements of bond-rupture forces can be used to reveal hidden features of molecular chemistry and structure."
FIGURE 1.In the UBC experimental setup, a personal computer controls the piezoelectric actuator, xyz translators, and output from a high-speed camera (a). A 200-W mercury-arc light source from Oriel Instruments is equipped with a narrow bandpass filter at 546.1 nm (b). The high-voltage piezoelectric power supply is microprocessor-controlled (c). The inverted microscope stage has a 5-mm-travel piezoelectric actuator, with a micropipette holding a test surface on the right and a stationary micropipette holding the probe on the left. Both are mounted on the xyz translators (d). A high-numerical-aperture objective lens is part of the inverted microscope (e). One CCD camera captures images at 30 frames/s (f); a second CCD camera captures images for tracking at rates as high as 1000 frames/s (g). An output image from the high-speed camera is shown (bottom right).
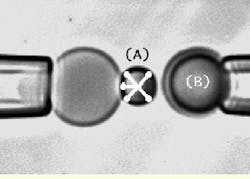
FIGURE 2.Details of the output image from the high-speed CCD camera show the left and right micropipettes, with a red blood cell appearing as a shadow inside the end of the left pipette. Each pipette has a microbead attached to it. The biomembrane force probe (BFP) on the left pipette (A) is kept stationary while the microbead test surface attached to the right pipette (B) is moved to make a soft contact with the BFP tip. It is then pulled away from the tip using precision piezoelectric control. The simulated X-shaped cursor tracks the image of the probe tip throughout the cycle of approach-touch-separation by video image processing at 1000 frames/s.
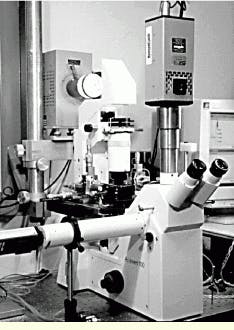
FIGURE 3.This is one of the three eyepieces of the inverted microscope within the biomedical system used at the University of British Columbia. The Cooke SensiCam camera observes the experiments through one of these ocular ports. Another camera port is located at the left side of the microscope. The microscope stage with the micrometer drives is positioned below and behind the microscope; the illumination source is placed behind this stage.