Biofeedback
Linescan camera, telecentric lens, and novel lighting automate slide reading
To enable high-speed blood lab diagnostic work, the Swedish company Amic developed the 4castchip, a polymer slide divided into micron-sized sections that each hold a drop of fluid that can be processed through a proprietary reading device (see photo). Amic holds worldwide patents on this point-of-care testing technology. To automate the dispensing process, Amic contacted BioDot—a supplier of system tools for the research, development, and commercialization of diagnostic tests—which had a machine platform for Amic but needed a customized vision system to ensure accurate dispensing. MoviMED, a vision-systems integrator, was engaged to develop the vision system.
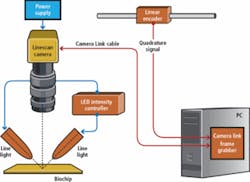
Markus Tarin, president and CEO of MoviMED, recognized the challenges to be faced by the vision system. Foremost was performing a single-scan image analysis of four different biochemical agents with different visual properties (representing a range of different fluids that could be scanned in commercial applications). MoviMED designed a prototype vision system for these simulated production conditions consisting of a high-precision linear slide with stepper motor and rotary encoder, a single-chip fixture, and a vertically mounted linescan camera (see Fig. 1).
“Since the aspect ratio of each chip is 1:3 and the features of interest are spread across the entire chip surface, this was a good fit for linescan,” says Tarin. “We wanted to avoid having to ‘stitch’ three square images together from an area-scan camera. We chose a linescan camera from DALSA--the Spyder-2, a 2k-pixel monochrome linescan camera with Camera Link interface, plus a power supply. This setup allowed us to try out different lenses and illumination, as well as scanning speeds.”
After dispensing biochemical solutions, the chips undergo a curing process that causes the liquids to dry. These dried spots have to be imaged. In testing, the contrast of the spots vs. substrate was virtually nonexistent. “The microstructure that was photoetched onto each biochip also had the tendency to function like thousands of miniature light guides, which seemed to interact differently with different biochemical agents,” Tarin says. Concurrently, the vision mechanics of the system were limited by the physical constrains around the dispensing head where the camera, lens, and illumination were mounted.
“Even though we were using a monochrome camera, we reaped marginal results using a red LED light,” Tarin says. “We achieved the best contrast using a white halogen light. However, the halogen source needed a fiberoptic setup to couple the light into the illumination. We had rapidly moving robotic motion with a fairly large envelope. The fiberoptic cable was too short and too stiff. Additionally, strong UV radiation was being emitted from the source, which was of great concern to Amic because it causes photobleaching in biochemical agents.”
No standard LED lighting was available with intensity comparable to the halogen line lights, so Metaphase Technologies was called on to develop custom line lights: 2-in. white, high-intensity LEDs with custom UV cutoff, intensity controller, and custom high-flex cables that suited robotic motion (see Fig. 2).
To overcome the varying optical properties of the different biochemical reagents, MoviMED returned to Amic. “Its biochemists came up with a solution that would generate more contrast on the chips. This was the only way to avoid having to use multiple light sources,” Tarin says.
This still left a question—would the current illumination also work for reading barcodes? This added the potential for overexposure due to the higher reflectance of the dissimilar surfaces. MoviMED set the system to programmatically change the exposure time for scanning barcodes and water-sensitive slides. “We had no issue with shortening the exposure time. In fact, it allowed us to increase the scanning speed for the other processes,” Tarin says.
The next challenge was resolution. “We were aiming at about 15-µm/pixel optical resolution.” Tarin says. “A 2048 (2k) linescan camera would have been sufficient if not for the unfavorable field of view (FOV) using off-the-shelf telecentric lenses. We chose a telecentric lens since our application required making precise geometrical measurements. This type of lens reduces perspective distortion within the depth of focus to an insignificant amount.”
To match the desired FOV of 26.5 mm with the camera sensor required a custom telecentric lens. Graftek Imaging was consulted, which, in turn, recommended obtaining a custom lens from Light Works. The resulting telecentric lens optical resolution is 12.8 µm/pixel. The FOV resulting from the primary magnification from the lens of x1.09 was 26.2 mm.
“The width of a biochip is 25 mm, so we had an extra 1.2 mm of ‘wiggle room’ that was needed due to manufacturing tolerances. The distortion is less than 0.3% over the total FOV of 26.2 mm. This would be a 78.6-µm error over 26.2 mm. Considering that the largest features we measure are only a few hundred microns, the error introduced due to optical distortion is negligible.”
To ensure the optical accuracy and repeatability, US National Institute of Standards and Technology traceable calibration targets from Edmund Optics were included. “We can measure 200-µm objects down to ±2-µm accuracy. This is attributed to the subpixel accuracy algorithms built into the vision development tools we use,” Tarin says.
Dispensing vision
To set mechanical tolerances of both dispensing and slide fixturing, the system must locate a fiducial marker that is a cross-hair mark on the chips. Initially, line lights were illuminating only the part of the cross hair that was parallel to the lights. Additionally, an unpredictable glowing halo effect appeared.
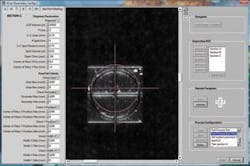
After trying to locate the fiducial dead center by using an edge algorithm and then using a pattern-match algorithm with limited success, MoviMED opted for an approximate region of interest with user correction. This requires the user to “dial” in the dead center with a superimposed cross hair on a magnified view of the fiducial. Once the center is marked, the software takes over and saves the location in memory. It can repeatedly locate the same center of the trained pattern on other chips, despite their variance in appearance. In statistical tests, Tarin says, this proved to be very repeatable and accurate. “We can compensate for up to ±500-µm displacement of each slide in either direction.”(see Fig. 3).
To ensure fast visual processing, a high-performance frame grabber with PCI Express interface from National Instruments (NI) was integrated. Tarin says, “The NI PCIe-1427 frame grabber seamlessly integrates with the custom application software we created using NI LabVIEW development software.”
An additional criterion for selecting this particular frame grabber was its programmable quadrature encoder interface, which allowed for easy interface to the high-precision linear encoder. A Renishaw encoder was chosen with a 0.1-µm resolution because the optical resolution of the system was 12.8 µm/pixel.
“In linescan camera applications it is always tricky to get a good ratio between the encoder resolution and the optical line resolution of the scan,” Tarin says. “Usually, this ratio turns out to be some fractional number that, in turn, causes the image to be distorted in the scanning direction. A one-dimensional image calibration has to be applied to correct for oversampling. In our case, we only had to divide by a factor of 128 and the line trigger would be precisely generated every 12.8 µm.”The reason for selecting both LabVIEW and an NI frame grabber is that no driver level software development has to be performed.
Presenting the system
The resulting machine is a stand-alone, four-axis motion system. Although loaded and unloaded manually, actual processing is completely automated (see Fig. 4). Fifty slides and four water-sensitive slides for calibration and one additional field (which carries the barcodes) are processed in each carrier tray at a scan rate of up to 50 mm/s. Any type of liquid can be dispensed onto the segments—blood, plasma, or drugs. Total scan time is about one minute for a nest of 50 slides, and every nest of slides is automatically calibrated for specific dispensing parameters.
“There is always a bit of mechanical play in any system, even on a small scale,” Tarin says. “We scan over every single slide before dispensing and correct for any slight misalignment. We know where everything should be positioned because of the fiducial marks, so we can generate a new dispensing pattern for each slide before ever dispensing” (see Fig. 5).
Individual pass/fail parameters are preprogrammed so the vision system automatically knows any “good dispense.” A “reject” dispensing head on the system drops a blue dot onto any chips that do not optically pass, making it easy for the operators to sort out bad chips.
“We have a very flexible measurement editor because we know the end user is going to process many different chips and will want to measure different things,” Tarin says. “It’s easy to reprogram rejection criteria to suite anything that may come up.”
MoviMED also developed an automated tip calibration and drop verification routine built into the dispensing system. The dispensing nozzle automatically adjusts in a way that is similar to how an inkjet printer goes through a calibration routine automatically whenever a new cartridge is installed. Anthony V. Lemmo, vice president of R&D at BioDot, says that this feature will be adapted to all future BioDot dispensing systems.
Features, advantages, benefits
Regarding the prototype vision system his company developed for Amic, Markus Tarin of MoviMED says that using water-sensitive slides for drop verification and tip calibration is a unique technology, specifically developed for the Amic project. Water-sensitive slides allow for exacting in-process machine calibration with respect to dispensing drop volume, as well as for drop placement, and his vision system was able to support this precision.
“In addition, using one facility to bring together the final integration of all automation and vision requirements allowed the process to be completed in only four months,” says Tarin. “This saved both our integrator, BioDot, and its customer, Amic, expense and time in system iterations. All that is left is the final certification of the system per the European Medicines Agency.”