Vendors benefit from document-validation systems
Often called inserts or outserts (depending if they are in the carton or on the bottle), folded documents are placed on the inside or outside of prescription medications and provide the consumer with a complete description of the drug, its properties, any health hazards associated with its use, lot dates, and other manufacturers codes. The documents can also include chemical formulas, barcodes, and graphical molecular diagrams that are printed, then folded, and attached to each medication. Although consumers rarely read these documents, the US Food and Drug Administration (FDA) mandates that they be included.
“In the past,” says Gary Parish of Complete Integration Systems (CIS; Indialantic, FL, USA; www.completeinspectionsystems.com), “the original documents were created in a Word format. Then images such as diagrams were scanned and pasted on and submitted to the FDA for approval. After the documentation was created, it was formatted in a single-page document and sent to the printer. To ensure the correct information was printed on the document, the original Word version was compared with the printed version before multiple documents could be printed.”
To ensure accuracy, the FDA created the 200% manual inspection rule, requiring two individuals to compare the two documents. Recently, the FDA has standardized the initial original New Drug Application submission format to an XML structure, but the conversion to the insert requires the same proofreading intensity to improve accuracy of the final printed material. But, unlike the standard “drug facts” on label copy, the type size, paper, and print color and contrast may still make reading difficult. “Now,” says Parish, “with the introduction of the FDA 21 CFR Part 11 requirements, regulations are in place for electronic records and signatures to also ensure pharmaceutical manufacturers must validate that the document and revisions are correctly identified as well as containing the correct copy.”
With documents so highly complex, proofreaders may spend many hours on each document to ensure that each one is correct. Add in multiple languages such as Japanese, Greek, or Chinese, and the task is almost impossible. Even with such people in place,” says Parish, “any human error such as a misplaced decimal point can result in costly recalls for the pharmaceutical manufacturer or vendors or a fatal dosage for the patient.”
CIS has developed a number of automated documentation systems and software suites that can scan, recognize, and automatically detect any errors between an original electronically generated document and a proof or press version. The suite of software, known as AutoProof Pro, consists of a number of different modules.
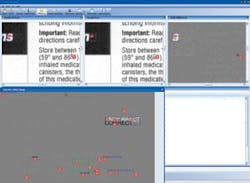
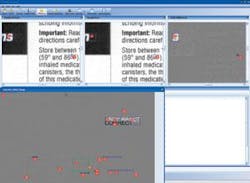
Most original documents are created by the manufacturer and sent to a printer. The printed inserts are shipped to the pharmaceutical manufacturer for incoming 200% inspection. To reduce the inspection process by up to 95%, CIS provides an image-compare module integrated with several input devices. For comparing materials up to 11 × 17 in., a flatbed scanner is used. For larger material, a sheet-fed scanner that can accommodate up to a 54 × 54-in. documents at up to 1200 dpi is used. After images are scanned, Docu-Match software auto-aligns the two images and finds any difference in text, images, and color. “The software automatically aligns the “master” and incoming material” for accurate comparison.
“In many cases,” says Parish, “the prepress version must be compared with a document generated as an Adobe Acrobat PDF file.” To do this, the PDF is converted to a bitmap and compared at the same resolution as the scanned materials. For comparing electronic revisions, CIS can compare each character, character for character, even though the format and location of the copy has moved. Results of the differences between digitized and original documents can then be compared on the PC’s monitor (see figure).
Many of these vendors produce drugs for clinical trials, for which small batches of labels must also be checked for accuracy. For these companies, CIS has developed a programmable vision workstation that uses a digital camera with the same software suite to check labels printed on laser, thermal-transfer, or dot-matrix printers. To do this, the camera captures each label to detect defects as small as 0.005 in. and uses optical character recognition to read multiple areas on the labels and compare the data with that from clinical-label generation program.
Says Parish, “We generally offer a totally integrated system including computer, cameras, scanners, and software to simplify validation and use. It has already been adopted by several pharmaceutical, prepress, and printing companies, which need to rapidly and accurately check highly complex documents.”