DETECTOR DESIGN IMPROVES positron-emission-tomography IMAGING RESOLUTION
DETECTOR DESIGN IMPROVES positron-emission-tomography IMAGING RESOLUTION
When used in positron-emission-tomography systems for cancer evaluations, circular crystal-scintillation-detector plates produce fewer parallax imaging errors.
By Kathy Kincade, Contributing Editor
One technique of nuclear medicine imaging, positron-emission-tomography (PET) imaging, is gaining support among clinical oncologists because of its ability to image tissue pathology, as well as anatomy and location. Unlike most other noninvasive imaging techniques, PET can
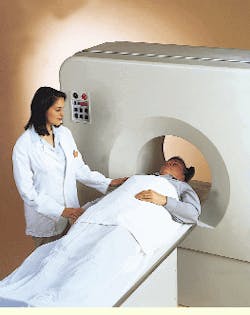
distinguish cancerous from noncancerous tissues and even determine how rapidly a cancerous lesion is growing. Such distinctions are extremely valuable in cancer staging and post-operative evaluations and in monitoring the course of radiation treatment and chemotherapy (see photo).
Positron-emission-tomograph imaging differs from other nuclear-medicine techniques in its use of an isotope that emits two simultaneous photons in opposite directions, thus eliminating the need for a collimator to detect the direction of the photons. The most common radiopharmaceutical used in PET imaging is fluorine-18 deoxyglucose (FDG), which has a tendency to concentrate in fast-growing, glucose-hungry tumors. As FDG decays, it quickly annihilates a positron that results in the emission of two gamma rays in opposite directions.
To capture and record each simultaneous photon-pair event--a process known as coincidence imaging--a series of crystal-scintillation-detector plates is positioned around the patient`s body. A matrix of photomultiplier tubes (PMTs) optically coupled to each detector plate captures the scintillations produced in the crystalline structure, converts them into electrical pulses, and then digitizes and uploads these pulses to an imaging workstation. Next, a custom reconstruction algorithm is used to create a three-dimensional (3-D) image of the activity distribution, including the physiology and location of the tissue or tumor being imaged.
"The key in PET is that you have detectors surrounding the [patient`s] body, and you take two points and electronically connect them, forming a line of response," says Joel Karp, associate professor of radiologic physics at the University of Pennsylvania (Philadelphia, PA). "To estimate and reconstruct a [PET] image, you have to measure the lines of response for many views or angles of the object. For example, if there is one single point of activity, all lines are going to intersect through that point. A distribution of activity can then be reconstructed as a linear superposition of many points," he adds.
Curved detectors
Positron-emission-tomography scanners contain many small scintillation crystals or a small number of large crystals that form position-sensitive detectors. The fewer the number of crystals implemented, the less expensive the scanner is to manufacture. This design approach was developed by Gerd Muehllehner, Joel Karp, and their colleagues at the University of Pennsylvania in the 1980s. In the mid-1990s, Muehllehner formed a commercial spin-off company called UGM Medical Systems (Philadelphia, PA). This company began marketing a PET scanner made of six large, flat crystal-scintillator-detector plates arranged in a hexagon-shaped setup to encompass a patient`s body.
Recently, UGM introduced an updated PET scanner that uses curved detector plates that obtain improved count rates and image resolution. This imaging system is being marketed and distributed by ADAC Laboratories (Milpitas, CA) under the name C-PET (see Fig. 1). It is manufactured by UGM and sells for $800,000 to $900,000.
The scanner`s detector-plate geometry is particularly important in FDG-based PET imaging because of its high-energy gamma-ray emission. Because these rays travel further into the crystal than do the lower energy rays associated with other nuclear-medicine cameras, much thicker scintillation plates are needed to obtain higher efficiency without sacrificing image resolution. However, a flat, thick PET detector plate runs the risk of generating parallax-imaging errors, which in turn can degrade the image resolution.
Curved detector plates perform better than flat detector plates do for two major reasons. First, circular detector plates surrounding the patient`s body produce fewer parallax-imaging errors. Because gamma rays from a single point can travel in any direction, some rays end up striking the flat detector plates at a more acute angle (lower angle of incidence) than others. This action creates greater spatial uncertainty. By curving the detector plates, however, more gamma rays are able to strike the plates at a normal (perpendicular) angle of incidence, thus reducing parallax errors and producing a less ambiguous image.
Second, curved detector plates also maintain their intrinsic spatial resolution better than flat detector plates do at higher counting rates. Scintillation light spreads out in a detector plate following a gamma-ray interaction, but the curved-plate geometry makes this light distribution more compact and keeps each event from interfering with the others. "You want the best spatial resolution, but you also want to measure as many simultaneous events as possible and as quickly as possible," Karp says. "This leads to higher image counts and better image quality for a fixed scan duration."
Another advantage of curved detector plates is increased incidence count rate (the detection of simultaneous photon events), which yields higher-resolution images. Because scintillation detectors are not always 100% efficient, and each event does not always have an identifiable ray partner on the other side, the number of recorded events in a detector is much higher than the number actually used for image formation. In fact, the number of events counted by each detector during a typical PET scan is approximately 700,000 counts/s to achieve a total coincidence counting rate of 50,000 counts/s. ©
Whereas the curved-detector-plate design has intrigued PET developers for some time, curved scintillation crystals have become available only in the last two to three years. Bicron (Newbury, OH), which manufactures a variety of nuclear-medicine components and instrumentation, has developed a proprietary, cost-effective technique for curving flat sodium iodide crystals without sacrificing crystal integrity.
Sodium iodide crystals offer several advantages over the bismuth germanate crystals used in other PET imagers, according to Karp. In particular, they are more economical--an important design consideration in the development of a commercial PET imager appropriate for clinical use.
Bicron supplies the detectors used in the C-PET system. Each of the six detectors contains one 1-cm-thick crystal and 288 PMTs, which together pick up about 6 million counts/s and offer a spatial resolution of 5 mm. The data from the detectors are multiplexed and digitized at a rate of 40 ns prior to being transmitted to the data-acquisition hardware. The sodium iodide-crystal detectors also enable the C-PET system to operate as a three-dimensional (3-D) imaging tool.
Another advantage of sodium iodide`s energy resolution is that it enables postinjection transmission scans using a cesium-137 source, which produces gamma rays. These transmission scans are needed to measure and correct for the attenuation in the patient`s body tissue; this attenuation greatly reduces the number of emission gamma rays that escape and subsequently hit the detectors during a PET scan. The sodium iodide detectors can discriminate the emission photons from the transmission photons, which speeds up the transmission scans and improves the accuracy of the attenuation correction. These factors are especially important during scans of a patient`s thorax, which includes lung and other soft tissues with various attenuation properties.
Hardware/software integration
In addition to the PET detectors, other crucial system hardware includes data-acquisition hardware, imaging workstations, and reconstruction software. The data from the detectors are delivered over a VME bus connection to a Sun Microsystems (Mountain View, CA) Model 5 workstation. This workstation determines the event position within the detector (to within 6 mm) and matches it to the corresponding point on the opposite detector. In turn, the workstation is linked via an Ethernet network to a Sun Microsystems Ultra 60 workstation, which is responsible for image reconstruction and display.
Reconstruction is handled by a custom iterative algorithm written in C using existing Fourier rebinning (resorting and compressing) and iterative reconstruction formulas. Unlike filtered back-projection algorithms (common in computed-tomography imaging), which back-projects lines of response and filters the data to focus on a single point, iterative algorithms are based upon the fact that the distribution of activity can be estimated and forward projected. This "guess" distribution is then compared with the measured and collected data. If the results are the same, the initial "guess" is correct. If the results are different, the ratio between the two data groups is forward-projected again, and another comparison is made. This method is continued until the correct image is obtained.
"The iterative approach does much better than the filtered back-projection when there is noise and low statistics," Karp says. "And for whole-body imaging, this makes more sense. If you scan larger portions of the body, you cannot collect as much data, so there is more noise." While the iterative algorithm is not new, UGM is the first to include it with a commercial system for routine clinical use, he claims.
System developers from UGM and the University of Pennsylvania have modified the original OSEM (ordered subsets expectation maximization) algorithm to handle the large data sets common to PET imaging (see Fig. 2). Intrinsic corrections for spatial detector distortion and normalization are performed prior to rebinning the data. The OSEM algorithm is a faster version of the expectation-maximization (EM) algorithm, which has been used in image reconstruction for some time.
"The EM algorithm was slow and would take hours to reconstruct," says Muellehner. "The ordered subset part was introduced byAustralian researchers to make it run faster, and the combination of faster computers and acceleration from ordered subsets make it fast enough for clinical acceptability." ©
After the data are collected, the algorithm is used to compress the data set from 11 Mbytes to about 1.6 Mbytes. This compression is important with whole-body scans because 22 Mbytes represents just one body position, and several body positions are needed for a full whole-body PET examination. Data acquisition and rebinning take about 40 minutes. Then, the compressed data are transferred from the Model 5 workstation to the UNIX-based Ultra 60 for 3-D reconstruction, which takes another 20 to 30 minutes.
Karp and another colleague at the University of Pennsylvania are now using the same curved-detector plate technology to develop more dedicated PET scanners, initially modifying the whole-body curved plate system for use as a dedicated breast imager. The breast scanner uses only two smaller detectors (400 sq cm) and 32 PMTs per detector. Early studies indicate that the dedicated design leads to improved detection of small lesions because of higher sensitivity and better spatial resolution. Sensitivity is predicted to be higher by a factor of five compared to the whole-body scanner, and image resolution is predicted to be less than 3.5 mm.
The CPET dedicated positron-emission-tomography scanner incorporates curved scintillate-crystal technology and coincidence imaging to achieve better image quality (less than 5-mm spatial resolution), lesion contrast, and anatomic localization of potentially cancerous lesions.
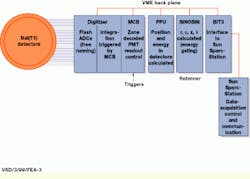
FIGURE 1. During C-PET scanner operation, the master controller board (MCB) receives a trigger that signifies a coincident event. Then, the digitized signals from two clusters of photomultiplier tubes are integrated and sent to the position processing unit (PPU) for position and energy calculations. The sinogram rebinning (SINOBIN) processes perform energy gating and calculates the projection coordinates. They achieve compact data storage and retain accurate information about the direction of the coincident events needed for three-dimensional image reconstruction.
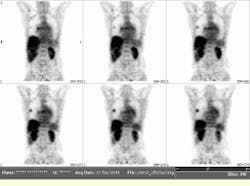
FIGURE 2. Representative coronal views were obtained from a fluorine-18 deoxyglucose oncology study performed at the University of Pennsylvania (Philadelphia, PA). The data for this study were acquired in less than 50 minutes, including both the emission scan and the transmission scan, which are needed for attenuation correction. Note the presence of two lung lesions.
The need for nuclear medical imaging
With the number of cancer deaths in the United States topping 550,000 in 1997 and an estimated 1.38 million new cases expected in 1998, the need for more accurate diagnostic imaging tools has become critical. The medical community is clamoring for new, noninvasive technologies that can reduce mortality rates and improve the quality of life for cancer patients.
Numerous imaging techniques already exist: computed tomography (CT), magnetic resonance imaging (MRI), optical biopsies, and x-rays, among others. But one of the most promising avenues for oncology is nuclear medical imaging. Because of its unique abilities to document organ functions and structures and provide direct views of disease physiology, nuclear imaging can help diagnosis, manage, treat, and even prevent serious diseases.
A key asset of nuclear medicine imaging is the radioactive tracer or dye that is injected into the patient prior to the scanning procedure. Depending on its dye compound, each radiophamaceutical is attracted to specific organs, bones, or tissues. As the compound begins to decay, it emits gamma rays that are captured by a nuclear medicine camera.