Harnessing UV-C LEDs for Advanced Inspection and Quality Control
What You Will Learn
- Mercury lamps are being phased out globally due to environmental concerns, prompting a shift to UV-C LED solutions with tailored wavelength peaks.
- UV-C LEDs enable precise fluorescence imaging in applications like adhesive inspection, food safety, and counterfeit detection, often replacing costly X-ray or destructive tests.
- The development of UV-C LEDs with peaks in the 265-285 nm range opens new possibilities for analyzing natural processes and chemical properties in various products.
- Machine learning models trained on UV spectral data can accurately differentiate authentic products from counterfeits, enhancing security and quality control.
Countries around the world are banning mercury-based illuminators. Mercury lamps are used in many machine vision applications such as inspecting currency for evidence of counterfeiting or sorting vegetables using chlorophyll fluorescence. Indeed, these broadband ultraviolet sources based on mercury have monopolized the market to the point where photoinitiators and fluorescent inks were developed to match the emission peaks of its spectrum.
With deadlines approaching, vision engineers are now starting to contemplate life without mercury, and in the very near future, a viable alternative will be required to replace these legacy fixtures.
LEDs are considered the natural successor. For every application that previously employed mercury, the question arises as to whether it can be achieved with an LED solution. However, this change can also be considered an opportunity to explore different technologies. LED solutions, for example, can potentially enable new applications that were previously unattainable.
Mercury bulbs are a great multi-wavelength source as they produce a set of discrete wavelength “lines.” These are generally high power, and a filter is used to selectively pass through the chosen wavelength, while blocking others when a single wavelength is required. This discrete nature leaves “dead zones” where mercury struggles. In the UV-C range, mercury has a predominant peak at 254 nm, but it struggles in the 265-285 nm range.
In response to this shortcoming of mercury, developers of LEDs have produced a series of LEDs with peaks in this range. This opens new applications where natural processes require light in this range (this could be a fluorescence or absorption feature).
Related: Optimizing Machine Vision Lighting for Food and Beverage Inspection
In this article, we will discuss how fluorescence using the UV-C wavelengths works and some current and prospective use cases for UV-C LEDs.
What is Fluorescence?
Fluorescence is the process where a sample is exposed to a specific wavelength (material dependent) that it absorbs, causing it to emit a longer wavelength. The absorbative/emissive properties depend on the chemical structure of the sample.
These absorbing wavelengths usually have a spectral width, where they will absorb wavelengths close to the peak and emit, but this is usually a much less efficient process. Given that fluorescence is already a “lossy” process (i.e., the light intensity produced is only a tiny fraction of what is used to cause it), using a non-optimized wavelength accentuates this fact. UV-C LED technology excels at any fluorescence that occurs in the 265-285 nm range.
Inspecting Adhesives with UV-C LEDs
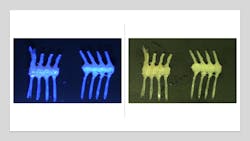
Engineers are using UV-C LEDs to inspect adhesives in a variety of sanitary products, such as diapers. They will often have optical brighteners added to them; these absorb radiation in the 280-360 nm range while emitting a bright blue color in the 430-460 nm range. This can be exploited to inspect the adhesive. The images above show the adhesive tab of a diaper under ambient light and when illuminated by 285 nm LED light. In the fluorescence image, one can see the strong blue line at the hinge point of the tab and the residue where the tab was stuck down, and on the tab itself. Fluorescence can be used to inspect the deposition of this adhesive during production.
The fluorescent properties of UV-C also can be used to inspect food. In fish processing, bone removal is a key step to ensure consumer safety. The standard screening process for bones in processed meat and fish is to use X-ray technology. However, this is very expensive, and fish bones are so small they won’t absorb enough X-rays to show up in scans. Because the bones are similar in color to the fish flesh in many instances, this can make visual inspection challenging.
Related: Condi Food Develops Hyperspectral Imaging Sorting Solution
Due to the collagen content in fish bones a fluorescence can be observed when using UV-C radiation. Collagen contains aromatic amino acids (mostly tryptophan, tyrosine, phenylalanine) with absorption maxima between 260–290 nm. These will fluoresce with a blue-violet color (380-400 nm) while the surrounding muscle will not flouresce.1 By using the appropriate filter, the bone can be highlighted, distinguishing it from the meat easily.
Absorbative Spectral Features
The advantage of fluorescent applications is that, despite requiring ultraviolet radiation, we observe the output in the visible range with a standard CMOS camera. However, along with UV LED development, advancements have also been made in ultraviolet camera technology.
Much like InGaAs cameras used in the SWIR vision sector, we can now target spectroscopic features in the UV range with the appropriate camera. Based on the material chemistry specific to the target application, we present case studies that would normally require a destructive lab-based test on a small sample. Instead, it is now possible to perform non-destructive techniques that can be applied to the entire batch of a product, ensuring 100% inspection within the process.
Inspecting Decaffeinated Coffee Beans
Take, for example, the decaffeination of coffee. Once the beans have been put through their decaffeination process (such as the Swiss water process or the carbon dioxide process), a batch of the beans will be destructively tested to measure their caffeine concentration.
Related: Applying Ultraviolet Lighting in Machine Vision Applications
Once this batch is below a certain level it is assumed the entire batch is then appropriately decaffeinated. Studying the absorption spectrum of caffeine in the UV range, it can be found to have a peak at 272 nm. If illuminated with a 275 nm light and inspected with a UVC-sensitive camera, the beans will reflect more light as the caffeine concentration decreases.
With careful training and development a solution can be developed to inspect the caffeine content of every bean of a batch and remove any that have not been appropriately decaffeinated. Thus, ensuring 100% batch quality.2
Using UV and Hyperspectral Imaging to Inspect Whiskey
UV-C machine vision lighting need not be confined to single wavelength features. The world of hyperspectral imaging is now being extended to the UV side of the spectrum. Already extensively used in the SWIR region, this allows for the full chemometric analysis of samples similar to that of a spectrometer combined with the spatial feature analysis of a camera.
In SWIR absorption peaks associated with compounds like water, proteins or oils are usually tracked, building a unique spectral fingerprint for each pixel in the image.
This fingerprint can be used to identify defects or track chemometric properties of our product.
Related: Machine Vision Inspection System Detects Parasites in Cod
Studies have shown the possibility of detecting counterfeit whiskey using UV spectrometry3. The flavor profile of whiskey is complex and comes from a group of compounds known as congeners. Each whiskey will have its own unique congener profile based on the production process (what grain is used, the malting process, the distillation process and barrel aging process).
Examples of these include:
- Vanillin (vanilla type flavor, combined with a spiced note) which is typically linked to the barrel aging process, where the lignin of the wood barrel is broken down into Vanillin.
- Ethyl acetate (fruity note, almost green apples) is a common ester continuously produced during barrel maturation through the interaction of acids and alcohol.
Counterfeit whiskey is made through the dilution of high value whiskey with less expensive, tasteless alcohol and adding coloring. While the alcohol-by-volume (ABV) is maintained, the congener profile cannot be faked easily.
This adulteration works due to the subjectivity of human taste, and the lack of experience with expensive whiskey. How many people have tasted a premium whiskey brand often enough to know what it should taste like? By recording a “golden reference” for each whiskey brand, we can test for major spectral differences in the UV spectrum, associated with counterfeiting.
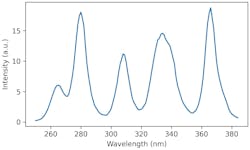
While not an ideal broadband hyperspectral source (the full range of wavelengths availability in LEDs is still limited), the proposed spectrum above does cover the full wavelength band of interest. Each sample of interest can be scanned and instantaneously assessed for tampering. Drastically increasing throughput compared to using a spectrometer on one bottle sample at a time.
Related: Deep Learning Tools Inspect Food and Organic Products
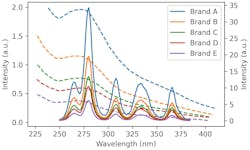
Here is the data presented in the aforementioned publication in which a number of authentic and counterfeit whiskeys were tested with a UV spectrometer (solid curves). Using this, we can also simulate what an arrangement of UV-C LEDs designed to simulate hyperspectral imaging would produce (dashed curves).
Using these curves we can train a rudimentary machine learning model. When tested, this model has the ability to correctly differentiate counterfeit from authentic samples 100% of the time. The caveat must be raised that this is simulated data on a small dataset. More widespread testing would be required to test this method’s robustness and accuracy.
Regardless of when the obsolescence of mercury lamps is actually enforced in various regions worldwide, it is clear that UV LED technology will, at some point, replace them. With efficiencies improving and prices dropping, UV-C LEDs are becoming a viable alternative, while also unlocking new applications that the fixed spectra of the mercury option does not allow.
References
- Łukasz Saletnik, W. S., et al. On the nature of stationary and time-resolved fluorescence spectroscopy of collagen powder from bovine achilles tendon. Int. J Mol Sci, 2023, 24(8), 7631. https://doi.org/10.3390/ijms24087631
- G. G. D'Souza J, Sundaram, N. UV induced photocatalytic degradation of caffeine using TiO2-H-Beta Zeolite composite. Minerals, 2023, 13(4), 465. https://doi.org/10.3390/min13040465
- Aylott R et al. Analytic strategies to confirm scotch whisky authenticity. Analyst, 1994, 119, 1741-1746. https://onlinelibrary.wiley.com/doi/10.1002/j.2050-0416.2010.tb00424.x
About the Author

Fabien Dubois
Fabien Dubois, PhD, an application engineer at ProPhotonix (Boston, MA, USA), completed his doctoral work in photonics in the Tyndall National Institute (Cork, Ireland). Since graduating, he has worked for FoodMarble Digestive Health (Dublin, Ireland) in the medical device industry and Tomra (Dublin, Ireland) in the food sorting industry. At ProPhotonix, Fabien uses his expertise in physics and machine vision to help customers find the best lighting solution for their application.